Research
Long-Term Phase 2 Trial Results
Published in Clinical Transplantation, August 2023
While kidney transplantation has traditionally required lifelong immunosuppression, an investigational stem cell therapy, FCR001, has been demonstrated to induce tolerance and eliminate the need for immunosuppression through the establishment of persistent mixed chimerism in a phase 2 clinical study. Real-world evidence demonstrated that kidney transplantation combined with non-myeloablative conditioning and FCR001 resulting in superior kidney function without increasing the risk of rejection, graft loss, or death among patients off immunosuppression.
Phase 2 Clinical Trial Results
Published in Transplantation, June 2023
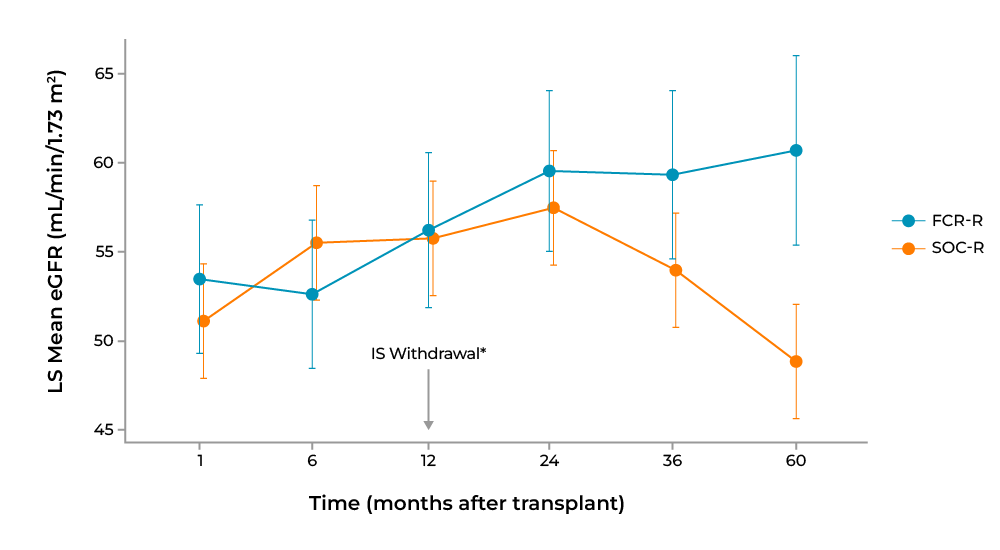
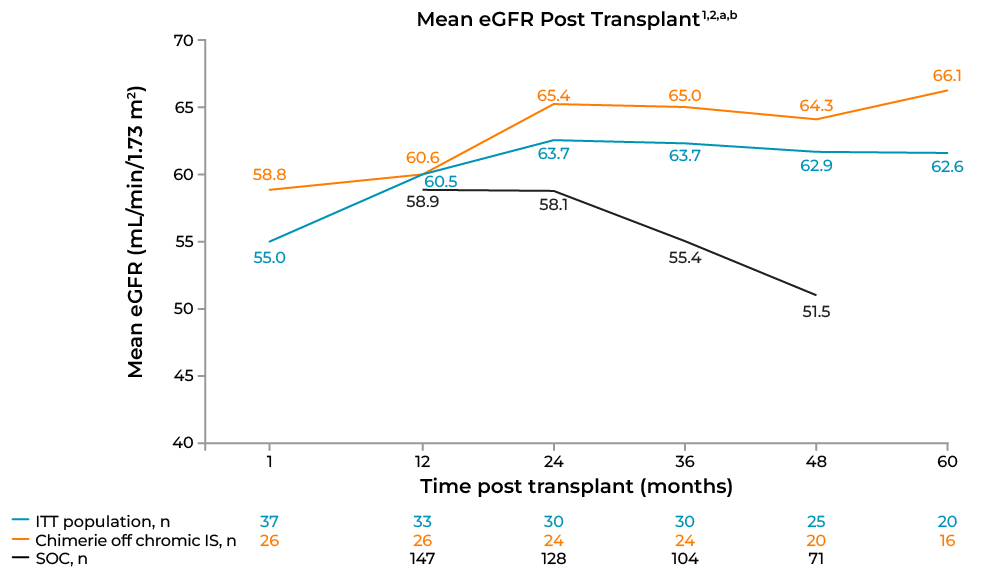
In the FCR001 cohort, a standardized measure of kidney function, estimated glomerular filtration rate (eGFR; measured by Modification of Diet in Renal Disease), among persistently chimeric participants improved over time compared with SOL control at all time points following 1 year post-transplant (Figure 4). BPAR occurred in 28.8% of SOC recipients at 2 years, 34.1% at 3 years, and 34.8% at 5 years post-transplant, whereas 0% of persistently chimeric participants in the FCR001 cohort off IS exhibited BPAR at all time points.
First Unrelated Delayed Tolerance Case
Published in Transplantation Proceedings, May 2021
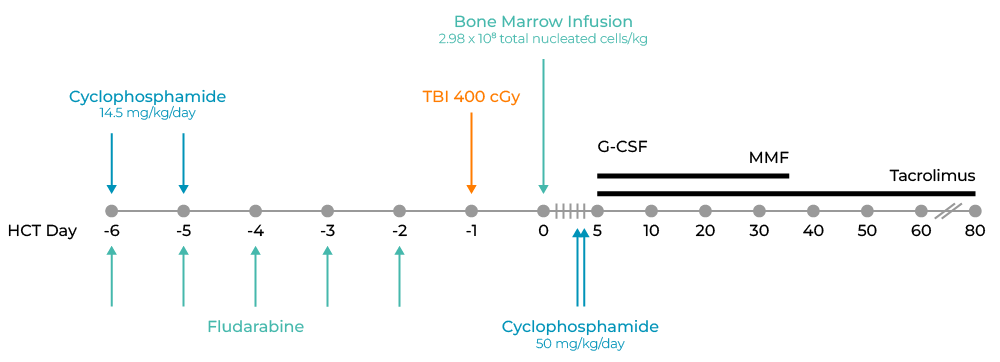
By day ±20 after HCT, he demonstrated donor engraftment with 100% full donor chimerism in unsorted peripheral blood cells. His post-HCT course was rather uneventful, and he was discharged on day +21 after HCT. At his most recent follow-up 12 months after HCT, he remained in complete remission with normal renal function, no evidence of active acute or chronic graft-versus-host disease, and complete immunosuppression withdrawal 6 months after the HCT. He maintained 100% full donor chimerism at 57 and 131 days post-HCT.
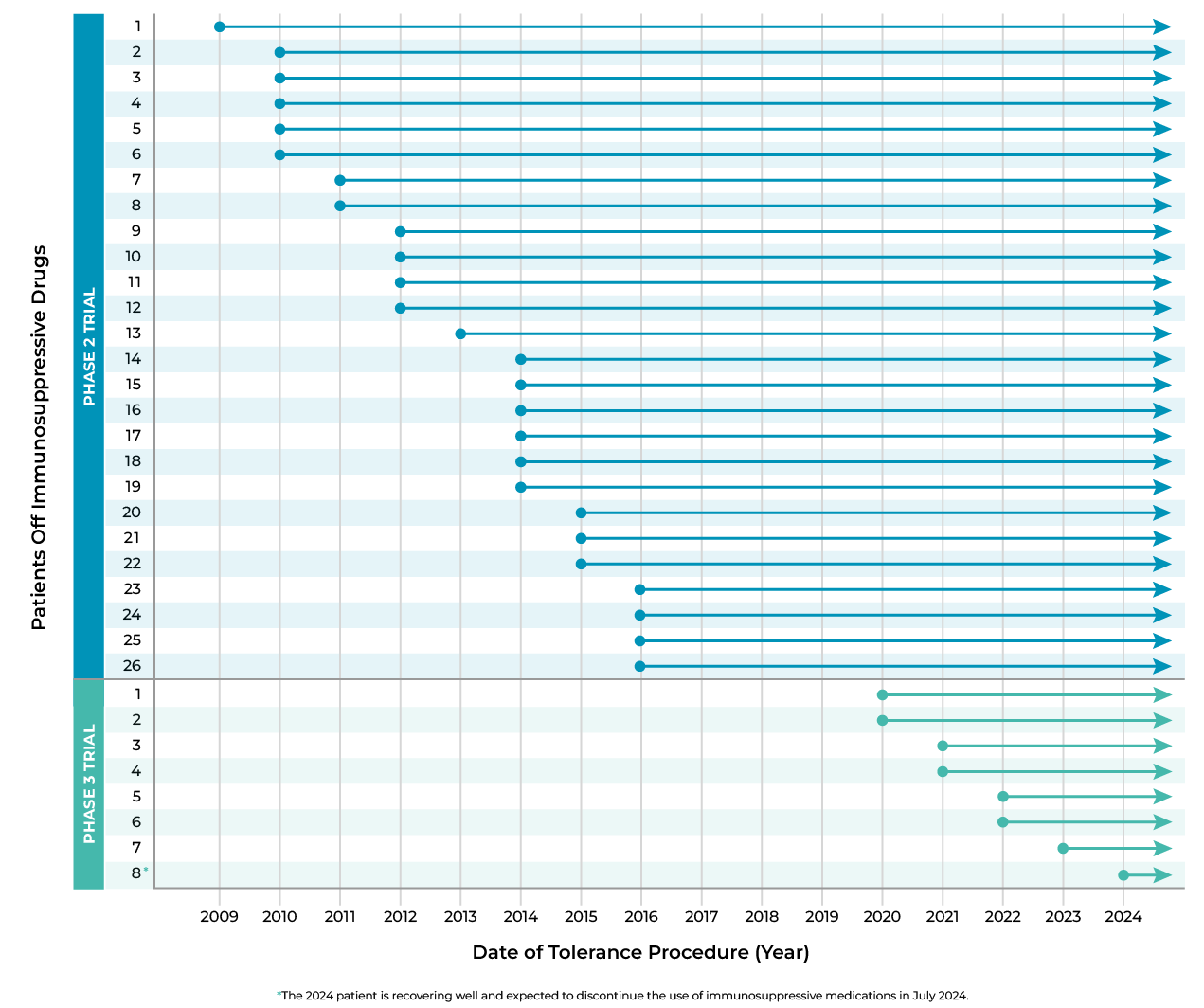
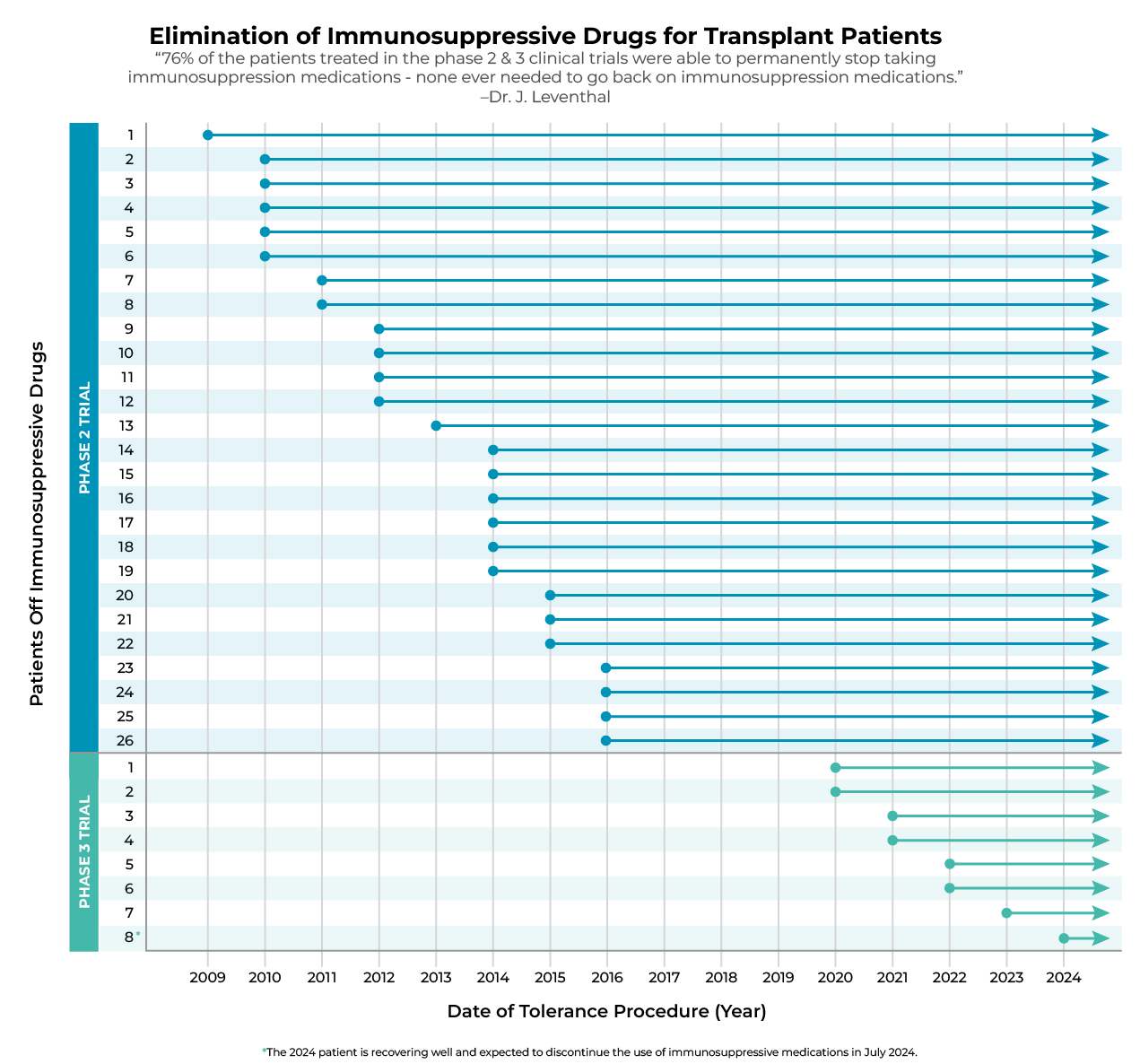
Elimination of Immunosuppressive Drugs for Transplant Patients
“76% of the patients treated in the phase 2 and 3 clinical trials were able to permanently stop taking immunosuppression medications—none ever needed to go back on immunosuppression medications.”
– Dr. Joseph Leventhal
This chart represents the patients who have stopped taking immunosuppression medications in phase 2 and phase 3 trials. Starting with the first patient in phase 2 to be withdrawn from immunosuppression in 2009, all patients who were withdrawn from immunosuppression in both phases of the trial have continuously been off of immunosuppression medications.
